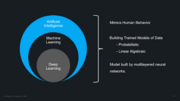
What Do Artificial Intelligence And Continuous Validation Have In Common?
Source:-https://www.meddeviceonline.com/ In 2011, the Center for Devices and Radiological Health (CDRH) initiated the Case for Quality, which provided guidance on how software validation processes can be improved and streamlined for the medical device, pharmaceutical, and biotech industries. By refocusing on patient and product safety, quality assurance, and data integrity, some suggest that validation documentation can be reduced (between 20% and 40%, in my experience) by applying automated testing and deployment. The benefits are accurate code, faster development processes, and reduced
Read more